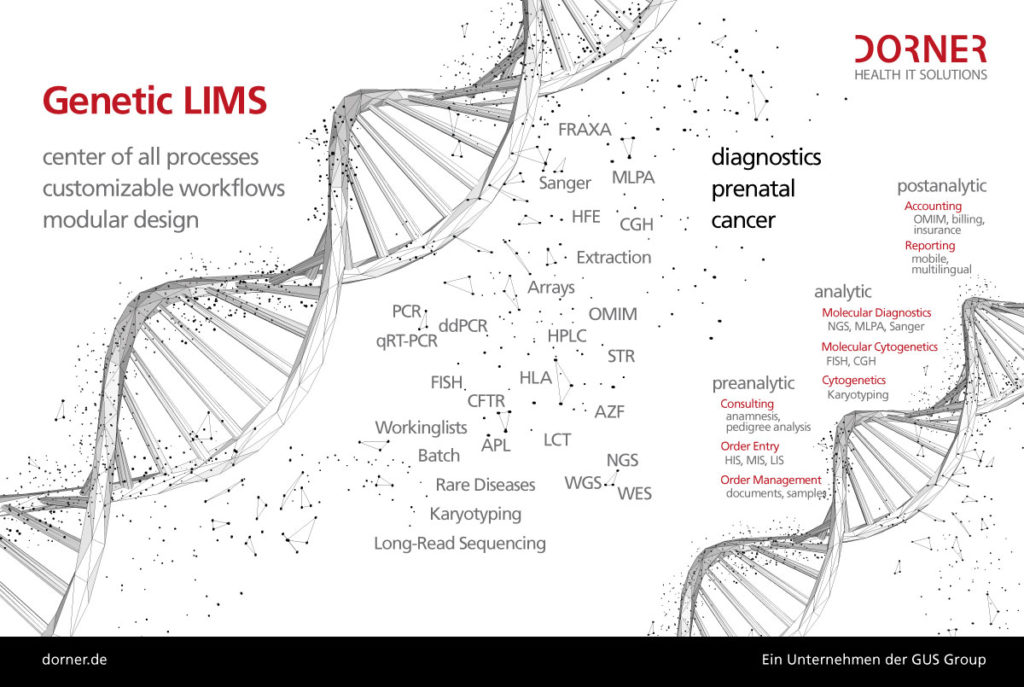
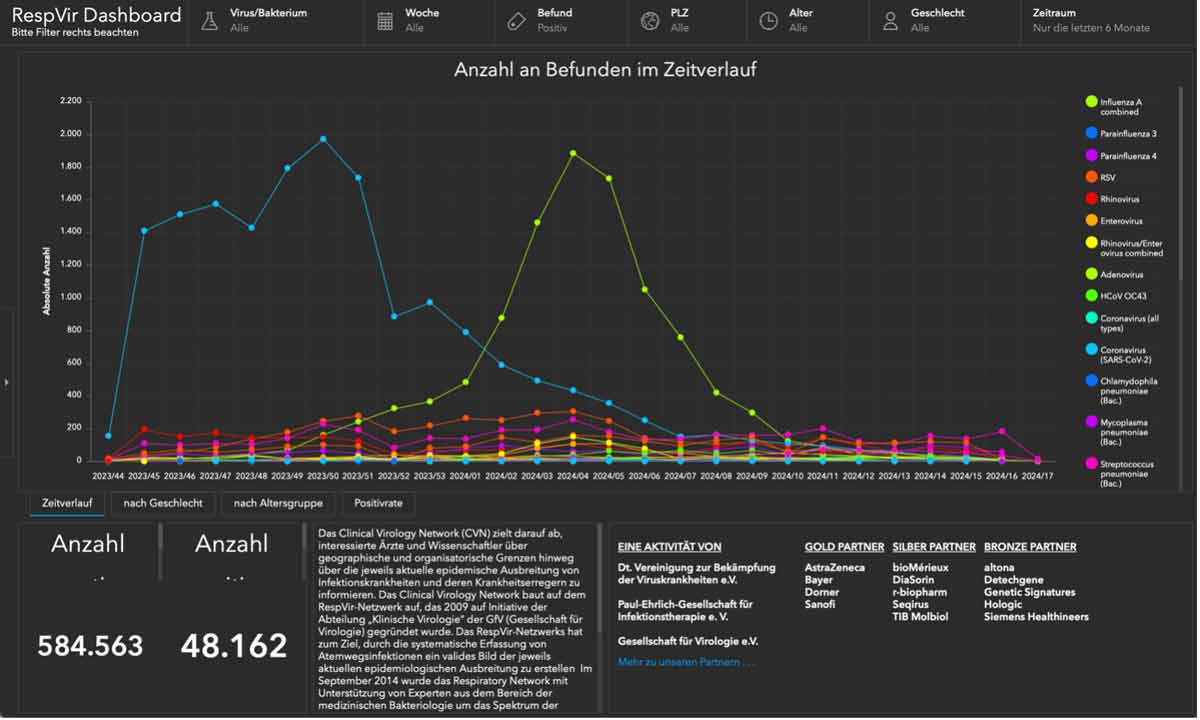
10 Essential functions of a LIMS in pediatric oncology
We have compiled ten essential functions that a LIMS in pediatric oncology and neuroblastoma research must fulfill in order to effectively contribute to research and improve patient care.
Today, the research community in pediatric oncology faces enormous challenges: a constantly growing number of research methods, an increasing number of samples and ever more extensive legal requirements. This complexity requires efficient organization and management of data and resources. This is where a Laboratory Information Management System (LIMS) can play a decisive role by helping to master these challenges and optimize the research process. A well-designed LIMS not only supports researchers in managing the daily flow of data, but also ensures compliance with strict data protection and security regulations. However, given the diversity and depth of requirements in this area, there are many aspects to consider when selecting and implementing a LIMS.
1. accreditation and audit trail
A comprehensive and integrated audit trail system is crucial for the accreditation of a LIMS. This feature ensures that every interaction with the system is seamlessly recorded. This includes changes, accesses and any kind of data manipulation. Such a system not only enables transparent and traceable documentation for internal quality controls, but is also essential to comply with regulatory requirements and standards. This ensures that the LIMS operates in accordance with industry best practices and guidelines, which is of paramount importance for research facilities and clinical laboratories.
2. data security and protection
In pediatric oncology research, ensuring data security and protection through a Laboratory Information Management System (LIMS) is critical. An effective LIMS should utilize advanced encryption technologies to ensure the security of patient data during transmission and storage. This includes the use of SSL/TLS for secure data transfers and database encryption to maintain the integrity of sensitive information. In addition, comprehensive compliance monitoring is required to ensure that the system always complies with the latest data protection standards and regulations, such as HIPAA or GDPR. These measures are essential to maintain the trust of data subjects and support research ethics.
3. data integration and management
In pediatric oncology, the efficient management of large amounts of data, including clinical data, research data and genetic information, is of central importance. An optimal Laboratory Information Management System (LIMS) should therefore enable seamless integration and effective management of this diverse data. Through advanced database technologies and user-friendly interfaces, such a LIMS enables researchers and clinicians to quickly capture, organize and retrieve data. This not only improves the efficiency of data analysis and research, but also contributes to the accuracy and quality of study results. An integrated LIMS is therefore a key tool to meet the challenges of the complex world of pediatric oncology research.
4. compatibility with existing systems
The interoperability of a Laboratory Information Management System (LIMS) with a variety of existing healthcare systems and technologies is essential for efficient and error-free data flow. An advanced LIMS must therefore be able to communicate seamlessly with a wide range of systems. These include patient records, hospital information systems, laboratory equipment such as NGS sequencers, specialized analysis software and even comprehensive enterprise resource planning systems such as SAP. For example, a LIMS should be able to import genetic data directly from a sequencer, integrate that data into the context of the electronic medical record, and ultimately synchronize with a system like SAP to manage resource management and billing information. This comprehensive interoperability is critical to maximize the efficiency of data processing while ensuring the integrity and accuracy of patient information.
5. adaptability and scalability
The adaptability and scalability of a Laboratory Information Management System (LIMS) are of great importance in the dynamic world of pediatric oncology research. An ideal LIMS must be flexible enough to adapt to specific, ever-changing research needs. This includes the ability to easily integrate new testing procedures or research protocols and handle growing data volumes. For example, the LIMS should be able to adapt to new genetic sequencing technologies while expanding the capacity to store and analyze increasing amounts of data. This flexibility ensures that researchers and clinicians can always work with the latest tools and methods to gain the best possible insight into neuroblastoma treatment and research.
6. promotion of cooperation
Promoting collaboration is a key element of an effective Laboratory Information Management System (LIMS) in pediatric oncology research. Such a system should offer features that facilitate the exchange and sharing of data between different research groups and institutions. This could be realized, for example, through a cloud-based platform that enables researchers to share data securely and in real time. In addition, integrated communication tools and shared databases could improve the coordination of studies and the flow of information. This type of networking and collaboration is crucial to efficiently share findings and advance research in the fight against neuroblastoma. A LIMS that supports such collaborative functions will contribute significantly to accelerating scientific discoveries and improving patient care.
7. integration of biobanking functions
Integrating biobanking functions into a Laboratory Information Management System (LIMS) is critical in cancer research, especially when working with biological samples. A LIMS tailored to the needs of pediatric oncology research should integrate advanced biobanking features such as sample tracking and management. This enables precise management of sample provenance, storage conditions, availability and usage history. For example, the LIMS could use barcodes or RFID tags for samples to ensure accurate tracking and localization. These features are beneficial for maintaining sample integrity and increasing the efficiency of research work by providing faster access to needed samples while ensuring compliance with regulatory requirements.
8. user-friendliness
Ease of use is a critical feature of a Laboratory Information Management System (LIMS), especially in the complex world of pediatric oncology research. A LIMS should provide an intuitive and easy-to-use interface that enables researchers and clinicians to use the system effectively, even without extensive IT knowledge. This could be achieved, for example, through clear and logical menu navigation, simple drag-and-drop functionalities and easy-to-understand visualizations of data. Interactive tutorials and a comprehensive help function within the system are also useful to make it easier for new users to familiarize themselves with the system. A user-friendly design of the LIMS contributes significantly to reducing the time spent on data entry and analysis and allows the focus to be placed on actual research and patient care.
9. creation of findings and analysis functions
The integration of advanced reporting and analysis capabilities into a Laboratory Information Management System (LIMS) is essential to increase the efficiency and accuracy of reporting in pediatric oncology research. A key aspect of this is the ability of the LIMS to seamlessly integrate all the data collected from different examinations into a single report. This includes the ability to create and save different report templates for different examination occasions. For genetic tests in particular, it is often necessary for all persons involved in the tests to be mentioned by name in the findings. An efficient LIMS should be able to automate this, for example by automatically extracting the names of the responsible personnel and the researchers involved from the project data and integrating them into the report. These functions not only considerably simplify the process of creating findings, but also ensure that all relevant information is accurately and consistently included in the documentation, which is of great importance for quality assurance and regulatory compliance.
10. compliance with regulatory requirements
Adherence to compliance requirements is essential for a Laboratory Information Management System (LIMS) in pediatric oncology. A LIMS must comply with both national and international regulations for clinical research and data protection. In Europe, for example, compliance with the GDPR (General Data Protection Regulation) is crucial to ensure the protection of personal data. In addition, European standards such as ISO 27001 are relevant for information security management systems. In the US, systems such as these must be HIPAA (Health Insurance Portability and Accountability Act) compliant to ensure data protection in the healthcare sector. Regularly adapting to such changing regulations and implementing security and privacy controls is essential to maintain the integrity of research and strengthen trust in the scientific community.
Good luck!
We wish you every success in your search for a suitable Laboratory Information Management System (LIMS)! Experience has shown that it is advisable to appoint a dedicated group of internal project managers who will approach this search with care and attention. Gather the specific needs of all departments involved to ensure that the new system meets the most important aspects of your work. Scheduling demo presentations and visiting reference facilities will not only provide valuable insight into how potential systems work, but will also help you better understand the philosophy and values of the vendors. This process is an exciting journey that will not only bring you a suitable LIMS, but also deeper insights into the vendor's collaboration and commitment to your long-term goals and success. We hope you will find the ideal partner for you!
Reference - Genetics LIMS in use
LabDia Laboratory Diagnostics at the St. Anna Children's Cancer Research Institute (CCRI), Vienna
One example of the successful use of our LIMS is LabDia Laboratory Diagnostics, whichworks closely with the St. Anna Children's Cancer Research Institute in Vienna. Here, our LIMS enables improved coordination and analysis of research data, which supports the development of new treatment strategies for pediatric cancers.
Oncology laboratory of the University Children's Hospital Zurich
Our LIMS system is already being used successfully in the oncology laboratory at the University Children's Hospital Zurich. Here, it has made a significant contribution to optimizing research processes and simplifying the management of patient data. The experts at the children's hospital particularly appreciate the intuitive usability and the system's ability to process large volumes of data efficiently.
Learn more about the DORNER Genetics LIMS
Would you like to learn more about how our specialized genetics LIMS can support your research in pediatric oncology? Visit our landing page for detailed information on the features, benefits and applications of our system. Discover how our LIMS simplifies genetic data management, fosters collaboration and helps you effectively meet the challenges of modern medical research. Click here to find out more and see how we can support your work.